Industries
Submit New Assignment
Insurance
Industrial & Manufacturing
Data Centers
Wind Turbines & Renewable Energy
Marine & Offshore
Healthcare
Education
Food and Beverage
Insurance
We assist insurers and adjusters on small or large cases to restore equipment back to pre-loss condition, ultimately, saving clients time and money.
Industrial & Manufacturing
We offer reconditioning and decontamination of specialized equipment. We understand industrial machinery and the costs and learning curves associated with new equipment.
Data Centers
We restore data centers based on the most cost effective measures, including an analysis of restore vs. replace.
Wind Turbines & Renewable Energy
Our team has successfully managed hundreds of projects on a wide range of makes and models, in over 60 countries around the globe, for more than 40 years.
Marine & Offshore
Our experts have been called in to assist with marine cases of all sizes, from small ships to some of the largest in the world.
Healthcare
Our engineers and technicians, many from the biomedical field, have provided guidance on recertification and reconditioning of medical equipment losses across the globe.
Education
We work with all involved parties to mitigate loss after an incident as quickly as possible and get school back in session.
Food and Beverage

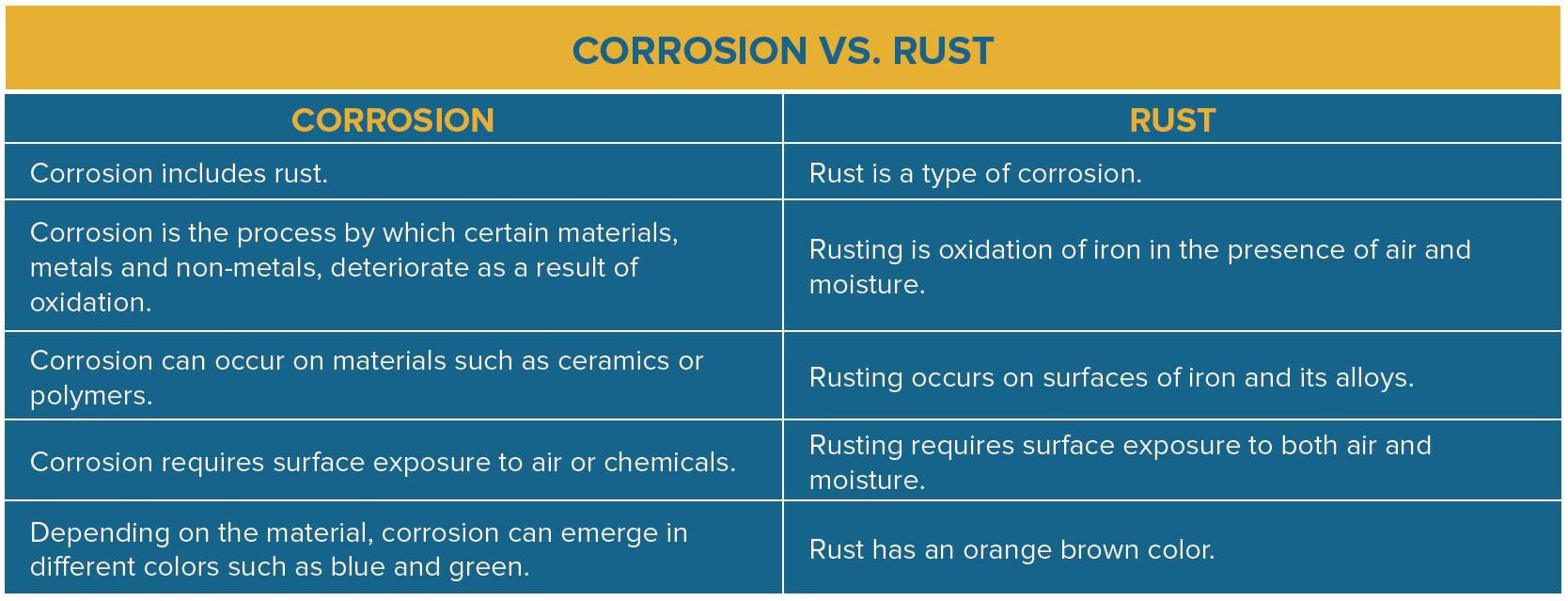 For more information on corrosion and rust, check out our whitepaper,
For more information on corrosion and rust, check out our whitepaper,